PHILADELPHIA – Flyer, a 70-pound golden retriever, lies patiently on her left side on an examination table as technicians scurry around, placing little sandbags on her legs and neck to keep her still. She’s getting chest X-rays to answer a critical question: Has a deadly bone cancer spread to her lungs?
When the session is over, Martha MaloneyHuss, a veterinarian at the University of Pennsylvania’s Ryan Veterinary Hospital, glances at the images. “I don’t see anything hugely obvious,” she says, “but we’ll see what the radiologist says.” Oblivious to the good news, Flyer hops down the hall on three legs, eager to find her owner.
After the 8-year-old retriever began limping last year, she was diagnosed with osteosarcoma, a painful, aggressive cancer that often strikes Great Danes, Irish wolfhounds and other large breeds. At Penn Vet, she got the standard treatment: One of her left legs was amputated, and she underwent chemotherapy.
Yet even as she adjusted to chasing squirrels, her prognosis was bleak. Most dogs die in about a year when the disease resurfaces in the lungs. The Penn vets recommended an experimental vaccine designed to prevent or delay the cancer’s return; Flyer’s owner was enthusiastic. The dog got three intravenous doses as part of a clinical trial and now returns to Penn periodically for X-rays.
“Every day I pray that she will stay cancer-free,” said her owner, Bob Street, who lives in Mullica Hill, New Jersey. “And that this treatment will work for other dogs and for people.”
Flyer is part of a burgeoning field called “comparative oncology.” It focuses on finding new ways to treat cancer in pets, mostly dogs, in an effort to develop innovative treatments for people and animals.
The growing interest in dogs reflects researchers’ frustration with the standard approach to developing cancer treatments: testing them in lab animals, especially mice. Mice don’t normally get cancer – it must be induced – and the immune systems in many strains of lab mice have been altered. That makes them especially poor models for immunotherapy, a rapidly growing field of medicine that directs patients’ own immune systems to fight their cancer.
Dogs, on the other hand, get cancer naturally, just as people do, and have intact immune systems.
“Genetically, you are a lot more like your dog than that mouse running around a cage in the lab,” said Nicola Mason, a veterinarian and immunologist who oversees the vaccine and several other canine trials at Penn’s School of Veterinary Medicine. “Where dogs really stand out is in the way they generate tumors and react to treatments, which is a lot like people.”
The vaccine Flyer got, for example, is now in an early trial for people after it showed impressive results in a previous Penn dog study. The company sponsor hopes to develop it for children and adolescents, who are more likely than adults to get osteosarcoma.
The dog and human versions of that cancer involve many of the same genes and are biologically similar. “What we learn in one species can be applied to the other,” said Robert Petit, chief scientific officer of Advaxis, the company testing the vaccine in people.
– – –
Across the country, medical and veterinary schools are partnering to find new treatments for malignancies from lymphoma and melanoma to brain and bladder cancer. The National Cancer Institute is overseeing canine trials at almost two dozen academic vet schools, and animal-health foundations are stepping up their support.
Immunotherapy is the latest rage in veterinary medicine, just as in human cancer treatments. Researchers hope studies in dogs can help explain why some people benefit from the approach and others don’t. Amy LeBlanc, director of NCI’s Comparative Oncology Program, said dog data can give drug companies “the necessary reassurance” to move forward with a promising treatment.
Not everyone is sold on the idea that pets will be that helpful for testing treatments in people. Many experiments are in the early stages. Laurence Baker, an oncologist at the University of Michigan who specializes in bone cancer, said that while he is “agnostic” about using pets for research, “in this area, as in too many areas of research, there is a lot more hype than success.”
Proponents of comparative oncology dispute that, saying the similarities of some cancers suggest benefits for both species. Cancer is the biggest health threat facing dogs, killing half of those 10 and older. “They are patients, not lab animals,” said Mason, whose office is filled with photos of dogs she has treated, including Aspen, a greyhound wearing bunny ears, and Bogie, a Great Dane.
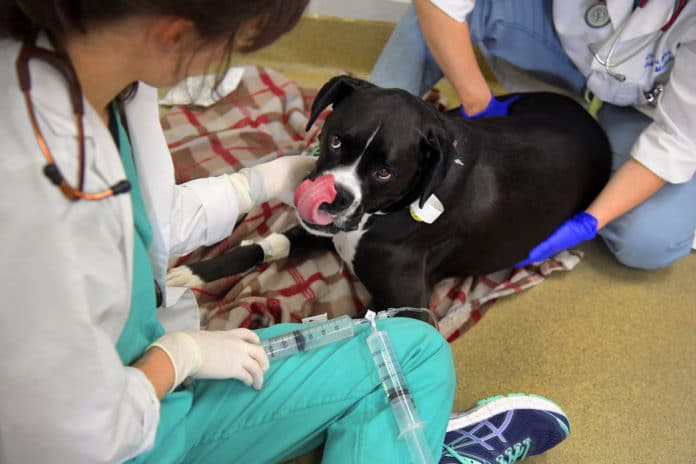
Most cancer drugs for dogs were developed first for humans. Take Harley, a striking 4-year-old black boxer with aggressive leukemia. He recently came to Penn for a treatment called CAR T-cell therapy, which has shown some promising results in trials for people with blood cancers. The immunotherapy procedure for dogs is similar to the human one: T cells, a key component of the immune system, are extracted, genetically modified in the lab to bolster their cancer-killing abilities, increased in number and then reinfused.
Mason began working on the treatment for dogs when she was a postdoctoral fellow in the lab of Carl June, a prominent researcher at Penn’s Perelman School of Medicine and a CAR T-cell pioneer. She consults with him frequently.
Owners of dogs in the Penn Vet trials get much of the care free, including treatments and follow-up visits. But there can be related expenses. In the osteosarcoma trial, owners are required to pay for the amputation and chemo.
If these canine treatments end up being sold outside clinical trials, they could carry hefty price tags. The human version of CAR T-cell therapy, for example, which is still in clinical trials, could cost hundreds of thousands of dollars once it hits the market. Scientists hope more progress will lower the cost for people and dogs alike.
– – –
The notion of using canines as a model for human cancers goes back decades. When veterinary oncologist Stephen Withrow was an intern at the Animal Medical Center in New York in the 1970s, he attended rounds at nearby Memorial Sloan Kettering Cancer Center and realized that animal and human medicine could help each other. “It opened my eyes,” he said.
Children with osteosarcoma have benefited from that insight. Withrow, who went on to found a major animal cancer center at Colorado State University, developed a surgical technique that allowed dogs with bone cancer to avoid amputation. He then collaborated with an orthopedic surgeon to adapt the limb-sparing technique for children.
In the 1990s, when an 11-year-old Colorado girl named Emily Brown was diagnosed with an especially complicated case of osteosarcoma, her doctors asked Withrow if there were any new canine treatments. He suggested an experimental therapy that Brown, now 30, credits with saving her life.
The sequencing of the dog genome in 2005 increased interest in comparative studies. “If the human genome is a deck of cards and you shuffle it, you end up with a rabbit – and shuffle it again, and you end up with dogs,” said geneticist Matthew Breen, whose lab at North Carolina State University’s veterinary school was involved in the sequencing project.
Marie Cary, who lives near Myrtle Beach, South Carolina, turned to Breen’s school a few years ago when her retriever, Gracie, developed a tumor on her upper jaw. After the cancer failed to respond to chemo, veterinary oncologists recommended an experimental treatment in which tiny particles were injected into the tumor with a light-sensitive compound called psoralen. X-rays then were used to target the particles, which responded by emitting ultraviolet light. That activated the compound and caused it to interfere with the tumor cells’ DNA and incite an immune response.
After a few rounds, the tumor disappeared; Gracie is now 10. The vet school is working with Duke Cancer Institute and the sponsoring company, which hopes to move the therapy into human trials.
Some comparative-oncology studies use cats for cancers such as oral malignancies and breast cancer, which have similarities to the human versions. But cats are used less frequently in studies than dogs, researchers say, because less is known about their tumors and they tend to get more stressed out interacting with people.
– – –
Osteosarcoma is diagnosed in about 10,000 dogs a year in this country, and researchers suspect that most die because the disease has already spread to their lungs. About 800 people also are diagnosed here annually, many of them children and young adults. Almost three-quarters are treated successfully with chemo; the rest die of advanced disease.
At Penn Vet, the vaccine trial in which Flyer was enrolled sprang from a collaboration between Mason and Yvonne Paterson, a microbiologist in the university’s medical school who survived breast cancer. The vaccine, which Peterson developed, uses genetically modified listeria bacteria to target cancers that are positive for a protein found in osteosarcoma, as well as in breast, gastric and other cancers. The bacteria, weakened so as not to cause illness, is designed to stimulate the immune system to recognize and eliminate any cancer cells remaining after chemo.
Mason launched the first trial in 2012 with 18 dogs. All got the standard treatment – amputation followed by chemo – then three vaccine doses. The first participant was Sasha, an American bulldog. She survived 738 days, compared with 423 days for dogs that had been treated in the past with amputation and chemo alone.
“I was a little worried about the possible side effects, so I put her in the ICU and sat with her for three days,” Mason said. “But it was anticlimactic. She did great.”
Many of the dogs did even better than Sasha, with median survival of 956 days. The vaccine now will be tested in a nationwide trial involving dozens of dogs.
In the meantime, Advaxis got the go-ahead from federal regulators to start an early-stage human trial with adults. Mason’s dog data was especially important in allaying concerns about possible heart damage. The company wants to launch a study next year for children with the aim of improving their treatment response and reducing relapse.
A few weeks after Flyer got a clean bill of health, Harley arrived for the CAR T-cell therapy for his leukemia. Mason had treated four dogs with lymphoma, but the first three had advanced disease and died quickly. The fourth survived for seven months.
To try for better results, Mason’s team changed the way it modified the T cells, bringing its procedures more in line with the techniques used for people.
Harley’s T cells had been withdrawn during an earlier visit, genetically altered and then multiplied. Now it was time for the 20-minute infusion. Erika Murphy of Franklinville, N.J., a teacher and mother of a young son, wept as she talked about the dog’s illness. “He’s our first boy,” she said.
Mason sat with him on a plaid blanket on the floor of a treatment room. MaloneyHuss administered the altered T cells via an intravenous catheter. Harley panted heavily but was calm, watching as a collie and other dogs strolled by.
The following week, he was back for tests. “He looks great,” Mason said, but the signs were mixed. “The cancer is coming back, but we are also seeing the T cells starting to expand. . . . Will they be able to start knocking back his cancer? We are all on tenterhooks to see.”